Compounder Fund: Cassava Sciences Sell Thesis - 19 Dec 2024
Data as of 16 December 2024
We first invested in Cassava Sciences (NASDAQ: SAVA) for Compounder Fund’s portfolio in August 2024. Our investment thesis for the company can be found here. In late-November this year, we completely exited Cassava Sciences. This article describes our Sell thesis for the company.
When we invested in Cassava Sciences, it was conducting two pivotal Phase 3 trials for its Alzheimers’ disease (AD) drug candidate, simufilam. Simufilam had shown promise in an earlier but smaller 24-month Phase 2 trial; in the trial, patients who were on simufilam experienced, on average, a slower rate of AD progression than those in the placebo group. But a Phase 3 trial for simufilam was necessary before it could be approved by the Food and Drug Administration (FDA) for commercialisation in the USA.
Besides having promising results in its Phase II trials, simufilam was a different class of drug than currently available treatment options for AD that are only mildly effective in controlling symptom progression. Simufilam also had a much better safety profile compared to two of the latest approved human monoclonal antibody drugs for controlling AD, namely, lecanemab and donanemab. If approved, simufilam could potentially be a game-changing drug for the management of AD.
Cassava Sciences was also shrouded in controversy at the time of our investment. This allowed us to pick shares up at a relatively cheap price compared to the potential value of the company we saw, based on conservative assumptions about simufilam’s market-share and the company’s profit margins on the drug, should it gain FDA approval. This presented what we thought was a highly-asymmetric risk/reward situation.
Unfortunately, it was not to be.
On 25 November 2024, Cassava Sciences released topline results for one of its Phase 3 trials named RETHINK-ALZ. The preliminary data were disappointing. They showed that simufilam did not meet either of RETHINK-ALZ’s co-primary endpoints, which are a slower rate of cognitive decline and a slower rate of functional decline. Table 1 below shows a summary of the data collected at the end of the 52-week RETHINK-ALZ study.
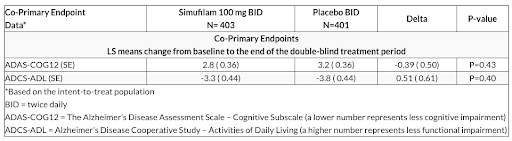
Table 1; Source: Cassava Sciences press release
The first co-primary endpoint for RETHINK-ALZ was to show a slower rate of cognitive decline based on the ADAS-COG12 test (the test is used to assess the cognitive impairment of patients with AD). The ADAS-COG12 test was administered to patients in RETHINK-ALZ at the start of the trial and at the 52-week mark. The results showed that patients on simufilam had a relatively slower rate of cognitive decline than the placebo group. But the slower rate of decline seen in the simufilam group was too low and is not considered statistically significant. With a P-value of 0.43 for the ADAS-COG12 endpoint, it means that there is a 43% probability that the observed difference in the two groups could have occurred by random chance. The P-value targeted for statistical significance is 0.05.
The second co-primary endpoint used the ADCS-ADL score to assess the functional impairment of RETHINK-ALZ’s patients. Again, the simufilam group showed, on average, a slower decline in functional impairment than the placebo group. But the slower rate of decline was also not considered statistically significant since the P-value is 0.4.
In order for RETHINK-ALZ’s results to have statistical significance, the rate of cognitive and functional declines in the simufilam group needed to be much slower than the placebo group compared to the trial’s collected-data. Alternatively, the P-value could also show statistical significance for similar data if the patient-population in the study was larger.
With the poor topline data from RETHINK -ALZ, Cassava Sciences’ management discontinued simufilam’s second Phase III study, REFOCUS-ALZ. This effectively ended any near-term hopes of FDA approval for simufilam. Management also decided to discontinue the open-label extension trials for simufilam; as a reminder, the open-label extension trials were meant for patients who have completed both of simufilam’s Phase 3 trials to continue taking the drug for the next 36 months, free of charge, if they so wish.
The outcome for simufilam was disappointing for us (and especially for patients with AD, who now have to continue waiting for a more effective treatment option for an ailment that carries a heavy toll on not just those afflicted, but also their loved ones). But we were not surprised; in our thesis, we mentioned that “only about 25%-30% of drugs in Phase 3 trials receive FDA approval.”
Unsurprisingly, Cassava Sciences’ stock price plummeted on the day RETHINK-ALZ’s topline results were released (it declined by 84%). With scarcely any hope for simufilam to gain FDA approval, we decided to sell Compounder Fund’s entire position in Cassava Sciences at an average price of US$3.99 per share, a far cry from our average purchase price of US$32 per share. In our investment thesis, we wrote:
“On the back of all this information, we decided to allocate around 0.9% of Compounder Fund’s portfolio into Cassava Sciences at our initial investment. We made Cassava Sciences a small position even though it has a highly asymmetric risk/reward profile, because there’s a material risk of the investment going to effectively zero very quickly if simufilam’s top-line results are poor or if the FDA eventually refuses to approve the drug.”
The loss for Compounder Fund’s investment in Cassava Sciences was a high percentage and came quickly. But its impact on the portfolio was small because of our effective risk-management approach of giving a low weighting to the position.
And here’s an important disclaimer: None of the information or analysis presented is intended to form the basis for any offer or recommendation; they are merely our thoughts that we want to share. Compounder Fund does not have a vested interest in any companies mentioned. Holdings are subject to change at any time.

