Compounder Fund: Cassava Sciences Investment Thesis - 20 Aug 2024
Data as of 18 August 2024
Cassava Sciences Inc (NASDAQ: SAVA), based in the USA, is a company in Compounder Fund’s portfolio that we invested in for the first time in August 2024. This article describes our investment thesis for the company.
Company description
Cassava Sciences is a clinical-stage biotechnology company. Its main therapeutic drug candidate is simufilam, a drug that can potentially slow the progression of symptoms of patients with Alzheimer’s disease. Currently, there are two ongoing Phase 3 clinical trials for simufilam and both aim to evaluate the safety and efficacy of the drug. There will be more on the trials in the “Simufilam’s Phase 3 clinical trials” sub-section.
Unlike all of Compounder Fund’s other investments, Cassava Sciences is not generating revenue at the moment, and it’s an investment we made because we believe the company is currently undergoing a special situation that provides a highly asymmetric risk/reward profile. As a reminder, we mentioned in our Owner’s Manual and website that “a small portion of Compounder Fund’s capital could also be invested in stocks that are undergoing special situations or have hidden asset values.” Cassava Sciences is the first such stock we have invested in for Compounder Fund since its inception. Consequently, this investment thesis will not have the same structure as our other theses.
The drug approval process
Let’s first start with a brief introduction on how drug-approval works. Before a drug is approved by the Food and Drug Administration (FDA) for commercialisation in the USA, it needs to be tested extensively via clinical trials, which are human-experiments designed to test the safety and efficacy of a drug. Typically, a drug will go through three phases of clinical trials and each phase can consist of multiple trials:
- Phase 1 is the first clinical trial that involves humans. It is usually a small scale trial, involving 20 to 100 individuals, with the primary end goal of determining the safety of the new drug candidate. According to the FDA, approximately 70% of drugs in Phase 1 trials move to the next phase.
- Phase 2 is a larger trial, typically with up to several hundred participants. This time, the trial’s primary goal is to study the efficacy and side effects of a drug. The FDA states that approximately 33% of drugs in Phase 2 trials successfully move to the next phase.
- Phase 3 is usually the last trial before a drug gets approved for commercialisation. It has an even larger number of participants – typically in the thousands – in order to get a more accurate picture of the efficacy of the drug. Only about 25%-30% of drugs in Phase 3 trials receive FDA approval.
- There’s also Phase 4, which is for investigating a drug’s long-term risks and benefits, dosage levels, and more. The patient population for a Phase IV trial is typically in the thousands. Drugs that have previously been approved may still be called by the FDA to undergo Phase 4 testing and surveillance.
Putting it all together, the likelihood of a drug receiving FDA approval after starting Phase 1 trials is only about 6%. This makes investing in pre-revenue, clinical-stage biotechnology companies – which is where Cassava Sciences is at – a high-risk proposition. Nonetheless, we believe the risk/reward profile for Cassava Sciences is highly favourable and we’ll explain why later.
Alzheimer’s disease and its pathophysiology
Dementia is a collective term referring to a range of symptoms that affect cognitive abilities in people, such as memory, reasoning, and thinking. Alzheimer’s disease (AD) is the most common cause of dementia, accounting for 60% to 70% of all dementia cases globally, according to the Mayo Clinic. AD is a degenerative disease of the brain that affects cognition, behaviour, and function. It is usually a progressive disease, which means a patient’s symptoms worsen over time. Patients with AD will present with a decline in memory, thinking, behaviour, and social skills. In late-stage AD, a patient’s ability to function will be impaired and a full-time caregiver may be needed.
According to the Mayo Clinic, approximately 6.5 million people in the USA aged 65 and older suffer from AD. In Singapore, the Ministry of Health estimates that 86,000 people aged 60 and above – or one in 10 – suffer from dementia with AD being the most common cause; the number of dementia patients in Singapore is expected to grow to 152,000 by 2030. Worldwide, it is estimated that 33 million to 38.5 million individuals have AD.
With the US population ageing (according to the United States Census Bureau, Americans aged 65 and older will increase from 49.2 million in 2016 to 77.0 million in 2034), the number of Americans living with AD is expected to climb substantially in the next decade or two as age is one of the biggest risk factors for the disease. The problem with AD today is that there is no cure and the management options to control the disease’s progression are limited. Before we dive into the current treatment options for AD, we will briefly explain, without getting too technical, how AD causes dementia.
The brain is made up of billions of neurons, which are specialised cells that transmit signals to each other and to different parts of the body. In normal ageing, the brain may shrink, but it does not lose neurons in large numbers. However, with AD, neurons stop working, lose connection with other neurons, and ultimately die. Researchers believe there are a few things that happen in the brain that can cause these neurons to stop functioning normally:
- Amyloid plaques: In simple English, these are proteins that clump together in between neurons and can disrupt cell function. The proteins come from the breakdown of a larger protein called the amyloid precursor.
- Neurofibrillary tangles: These are abnormal accumulations of a protein called tau that collect inside neurons. Tau proteins are present in healthy neurons but are usually attached to a part of the neuron called microtubules. But in AD, tau proteins are detached from microtubules and stick to other tau proteins forming tangles, thus blocking neurons’ ability to communicate.
Current treatment options for Alzheimer’s disease
As mentioned earlier, there is currently no cure for AD. In addition, only a handful of drugs have proven to be mildly effective in controlling symptom progression.
The first group of therapies are an older generation of drugs that are collectively called cholinesterase inhibitors. These drugs help to prevent the breakdown of a chemical messenger in the brain, thus improving neuron communication. The first FDA-approved cholinesterase inhibitor is tacrine, which was approved in 1993. Tacrine is no longer in use (withdrawn in 2013) and there are now three cholinesterase inhibitors that are commonly prescribed for AD, namely, donepezil, galantamine, and rivastigmine. The problem with cholinesterase inhibitors is that only a limited number of patients will respond to the treatment and there is also limited control of symptom progression.
The next group of therapies are NMDA receptor antagonists. NMDA stands for N-methyl-D-aspartate, and NMDA receptors are involved in cell communication in the brain. The NMDA receptor antagonist that is prescribed for AD is memantine, which was approved by the FDA to treat moderate-to-severe AD in 2003. This therapy can also be used in combination with cholinesterase inhibitors.
In 2021, a new class of drugs, with the first being aducanumab, was approved by the FDA for managing AD. Aducanumab is a human monoclonal antibody that aims to break down amyloid plaques in AD patients. There was some controversy surrounding the FDA approval of aducanumab because of the high incidence of severe side effects associated with the drug. Around one-third of patients in aducanumab trials developed ARIAs (amyloid-related imaging abnormalities) that were captured by MRI (magnetic resonance imaging) scans on the brain; the ARIAs were caused by swelling or bleeding in the brain. Earlier this year, Biogen, the biotech company behind aducanumab, announced the discontinuation of the drug for treatment of AD.
There have also been two new human monoclonal antibody drugs targeting amyloid plaques that received full FDA approval in recent months for the treatment of early AD. The two drugs, lecanemab and donanemab, have shown some efficacy in slowing the progression of AD symptoms in patients. Lecanemab (brand name Leqembi) was approved in July 2023, while donanemab (brand name Kisunla) was approved in July 2024. Both lecanemab and donanemab present a minor inconvenience to patients in that they are delivered via intravenous (IV) infusion. The major downside to both these drugs is their propensity, in a similar manner to aducanumab, to cause ARIAs in patients. Frequent MRI brain scans are needed to monitor patients who are on these two drugs.
Cassava Sciences’ simufilam
This brings us to Cassava’s Sciences’ drug candidate, simufilam. Simufilam comes in the form of an oral pill, unlike lecanemab and donanemab, which require IV infusions. Simufilam’s mechanism of action is also different from the other classes of AD drugs we discussed above. It targets an altered form of a protein called filamin A. In healthy humans, filamin A links to other proteins and stabilises them. But in AD, the brain has an altered form of filamin A because of amyloid-beta-42 proteins. Earlier, we mentioned that AD occurs because of amyloid plaques in the brain. Well, the most toxic form of amyloid proteins is amyloid-beta-42, which alters the shape of filamin A, which in turn enables neurotoxicity to occur in the brain, including the aggregation of tau proteins and the disruption of neuron function. Cassava Sciences’ management believes simufilam binds to altered filamin A, restoring the protein’s proper shape and function.
Simufilam’s Phase 1 and initial Phase 2 clinical studies
Simufilam has taken a long time to reach its current status. Cassava Sciences began early studies related to simufilam around a decade-and-a-half ago. It then successfully filed an Investigational New Drug (IND) application with the FDA for simufilam in 2017.
The first clinical trial for simufilam occurred in the same year of its IND filing in the form of a Phase 1 trial in healthy human volunteers to test for the drug’s safety profile. The trial was a success as the drug was deemed safe with no treatment-related adverse events. In 2019, Cassava Sciences completed an open-label Phase 2A study of simufilam. This was a small study of 13 patients with mild-to-moderate AD who were treated with 28 days of simufilam. The trial suggested that simufilam led to improvements in the biomarkers of AD pathology. In September 2020, the company reported the final results of simufilam’s Phase 2B study. This was a clinical study that was funded by the NIH (National Institutes of Health), in which patients were treated with 50mg or 100mg of simufilam twice daily and compared against patients who took a placebo. In this study, patients who took simufilam showed slower symptom progression versus those who took a placebo.
These were the early studies that enabled Cassava Sciences to invest in a much more extensive and robust Phase 2 trial that was done over 24 months.
Simufilam’s 24-month Phase 2 study
Cassava Sciences embarked on its extensive 24-month Phase 2 clinical trial of simufilam in March 2020. The results were reported in July 2023 and they are impressive. The trial started with 157 patients being given 100 mg of simufilam daily for 12 months. Patients were then randomised into two groups. The first group continued receiving simufilam for another 6 months, while the second was given a placebo for the same time period. The trial was double-blind, meaning both patients and the trial administrators were not informed of who was given simufilam or the placebo to reduce any bias when recording results. Ultimately, 78 patients were randomly placed in the simufilam group and 77 were placed in the placebo group. Patients in the trial were then given the ADAS-Cog 11 test, which is a list of questions and tasks used to determine the cognitive ability of an AD patient (ADAS-Cog 11 refers to the cognitive subscale of the Alzheimer’s Disease Assessment Scale that comprises 11 modalities).
The simufilam group recorded a 0.9 point decline in the ADAS-Cog 11 test while the placebo group reported a 1.5 point decline. This suggested that the simufilam group’s rate of symptom progression was 38% slower than the placebo group. The result also compares favourably with monoclonal antibodyAD drugs; when the simufilam trial results were announced, Cassava Sciences’ Chief Medical Officer, Dr James Kupiec, said (emphasis is ours):
“Monoclonal antibody drugs have slowed cognitive decline by 35% or less in early Alzheimer’s patients in large Phase 3 trials over 18 months. In this context, we believe results of our 6-month study are encouraging, despite vast differences in patient selection and the design and results of our randomized withdrawal study compared to large Phase 3 trials.”
More impressively, simufilam was actually much more effective in patients with mild AD. The mild AD patients were defined as those with MMSE (mini-mental state examination) scores of 21 to 26. The MMSE is similar to the ADAS-Cog 11 test in that it involves a list of questions that evaluates a patient’s cognitive ability. There were 40 patients in the simufilam group with mild AD, and 36 in the placebo group. In the simufilam group, mild patients showed a 0.6 point improvement in the ADAS-Cog 11 test after six months, while the placebo group reported an average 0.6 point decline. This result suggested that simufilam is more effective than any current AD treatment options available. In addition, the drug was shown to be well tolerated with no drug-related serious adverse events, unlike some of the aforementioned medications that are currently being used to treat AD. Importantly, the analysis was carried out by Pentara Corporation, a third-party consulting firm specialising in complex statistical analysis of clinical trial data, and the clinical data was captured directly into an electronic data capture system managed by an independent data management vendor.
Although the results of simufilam’s Phase 2 clinical trials are promising, they are not conclusive. The trial was still relatively small in size, which opens up the possibility that the results are not statistically significant. Put another way, the positive outcomes may be due to chance.
Simufilam’s Phase 3 clinical trials
This brings us to simufilam’s Phase 3 trials, which were initiated in the third quarter of 2021. At the start of this thesis, we mentioned that there are currently two Phase 3 studies being done simultaneously. Both are randomised control trials, which compare placebos versus different dosages of simufilam. The main goal of the trials is to further evaluate the safety and efficacy of the drug.
The first trial, called RETHINK-ALZ, is a 52-week trial comparing patients who are put on 100mg of simufilam twice a day with another group of patients who are taking a matching placebo. The patients are randomised 1-to-1 into the simufilam group and the placebo group. The second trial, named REFOCUS-ALZ, is a 76-week trial. It is comparing three groups of patients who are randomised 1-to-1-to-1. The first group are on 100mg of simufilam taken twice daily, the second group are on 50mg of simufilam taken twice daily, and the third group are on a matching placebo. The administration of the trials are outsourced to Premier Research, an independent contract research organisation (CRO). Both trials are fully enrolled, with around 1,900 patients in total randomised into the studies (around 800 for RETHINK-ALZ and around 1,100 for REFOCUS-ALZ). Among the total patient cohort, 70% of them have mild AD (MMSE scores of 20 to 27). The last patient visits for RETHINK-ALZ is expected in October 2024 and the first read out of the trial’s initial data is expected to occur near the end of this year. Meanwhile, REFOCUS-ALZ expects its last patient visits in May 2025, with the initial data to be released around the middle of 2025.
Although no information related to the efficacy of simufilam in the Phase 3 trials have been released thus far, safety reports have been made known and it has been concluded that the trials are safe to continue. Cassava Sciences has held two routine meetings with the Data Safety and Monitoring Board (DSMB) in September 2023 and March 2024 for simufilam’s Phase 3 trials. In both meetings, the DSMB, which comprises independent experts who are responsible for patient safety in clinical studies, gave the greenlight for the Phase 3 trials to continue as planned without any modifications. Importantly, safety data from simufilam’s Phase 3 trials to-date have suggested that the drug is not associated with ARIAs, which are the serious side effects related to lecanemab and donanemab.
Cassava Sciences also announced no-cost access, open-label extension trials in late-July 2024 for simufilam. The open-label extension trials mean patients who have completed the Phase 3 trials will be allowed to continue taking simufilam for the next 36 months, free of charge, if they so wish. The data from the open-label extension trials will then be analysed as part of further research into simufilam. A positive development for Cassava Sciences is that 89% of patients in the ongoing Phase 3 trials have elected to continue with the open-label extension trials. In other words, an overwhelming majority of AD patients in simufilam’s Phase 3 trials have chosen to continue taking the drug even after their participation in the trials ends.
Simufilam’s phase 3 clinical trials have patient groups that are much larger in size than the Phase 2 trials. This should provide sufficient data for Cassava Sciences to seek FDA approval for simufilam if the Phase 3 results are favourable.
The strength of Cassava Sciences’ balance sheet
Cassava Sciences has no revenue and deep cash burn – its operating cash outflow in 2022, 2023, and the first half of 2024 was US$77.5 million, US$82.0 million, and US$37.4 million, respectively. It is thus important that Cassava Sciences has sufficient cash on its balance sheet to complete simufilam’s Phase 3 trials.
We invested in Cassava Sciences a few days before management released the company’s 2024 second-quarter results on 8 August 2024 and held a conference call discussing them. The latest financials we had for the company at the time of our investment showed that it had US$124.2 million in cash on its balance sheet as of 31 March 2024 and no debt. Moreover, Cassava Sciences announced on 8 May 2024 that it had raised US$123 million in net proceeds from the cash-exercise of warrants; essentially, the company sold 5.7 million new shares of itself at US$22 apiece. Although the cash-exercise of the warrants diluted existing shareholders, we think it was still a good move by the company to beef up its balance sheet.
As of 30 June 2024, Cassava Sciences had US$207.3 million in cash and no debt. For the second half of 2024, management expects the company’s operating cash outflow to be between US$80 million and US$90 million. This includes a US$40 million loss contingency for a potential settlement with the Securities and Exchange Commission (SEC) that is related to investigations on the company that we will discuss in the “The things in favour of simufilam” section. All told, Cassava Sciences’ management expects the company to end 2024 with US$117 million to US$127 million in cash. We believe, as does management, that this is a sufficient amount of cash to fund the remainder of simufilam’s Phase 3 trials.
A potential fraud?
Up to now, we have only discussed the positive aspects of Cassava Sciences. But there are important controversies involving the company that we want to highlight.
It all started in August 2021. Back then, Cassava Sciences was hit with a Citizen Petition claiming that there were data anomalies and manipulation in its early studies of simufilam. A Citizen Petition is a way for individuals to petition the FDA to take action against a drug. According to the petition, Cassava Sciences had up to then, reportedly collected US$250 million in public fundraising via stock sales and US$5 million in grants from the NIH. The petition claimed that Cassava Sciences’ management was defrauding shareholders and the institutes who offered the company grants. It also claimed that the company was putting patients at risk by continuing with trials for simufilam. The petition requested that the FDA halt ongoing trials of simufilam until an audit is done. When the petition was filed, the identity of the whistleblowers were not publicly revealed, but it was known that they were short-sellers of Cassava Sciences’ shares who stood to gain financially if the company’s stock price fell.
In February 2022, the FDA declined to halt simufilam’s ongoing trials because of the Citizen Petition, but also said it was continuing to look into the allegations. Then earlier this year in March, media reports on the findings of a September 2022 FDA investigation on Dr Wang Hoau-Yan surfaced. Wang is a scientist whose research on simufilam’s mode of action in combating AD – the binding to altered filamin A proteins – formed the basis for Cassava Sciences’ development of the drug. He is also one of the scientists who was leading the research on simufilam in its early Phase 2 trials. According to the FDA’s investigation report, Wang may have engaged in poor laboratory practices and conducted improper statistical tests, potentially resulting in inaccurate data. The report calls into question the legitimacy of the results of early research on simufilam as well as those from its early Phase 2 trials.
Besides the FDA, the SEC and the Department of Justice (DoJ) also investigated the company. In June this year, the DoJ’s investigation caused Wang to be indicted for scheming to defraud the NIH for a total of around US$16 million in grants awarded to Wang and Cassava Sciences between 2017 and 2021. Wang is accused of making false statements about the mechanism of simufilam and the improvement in certain biomarkers and indicators in early-stage clinical trials for the drug. He is also accused of falsifying the results of his research to NIH, such as manipulating images of Western blots (a laboratory technique to identify proteins). Wang is charged with one count of major fraud, two counts of wire fraud, and one count of false statements. If convicted on all charges, he faces the possibility of up to 55 years in prison. These are serious allegations and put into question not just Wang’s integrity, but the legitimacy of simufilam’s mechanism of action and clinical efficacy. Making matters worse is the fact that Wang stood to gain financially if Cassava Sciences’ market capitalisation reached certain thresholds. Wang was part of a cash incentive plan the company established in August 2020 that would see cash bonuses be paid out each time Cassava Sciences’ market capitalisation reaches a certain specific threshold (the threshold starts at US$200 million and ends at US$5 billion) and stays there for at least 20 consecutive trading days. This gives Wang incentives to potentially falsify simufilam-related results to juice up Cassava Sciences’ market capitalisation.
Cassava Sciences’ stock price fell by 35% to US$12.35 on the day after Wang’s indictment. The company has tried to distance itself from Wang, announcing a day after the indictment that Wang was a former science advisor and has no involvement in simufilam’s Phase 3 clinical trials. The company also mentioned in a separate regulatory filing that it had removed Wang as a participant in the cash incentive plan prior to his indictment.
Just 20 days after Wang’s indictment was announced, Cassava Sciences’ founder and long-time CEO, Remi Barbier, resigned alongside Dr Lindsay Burns, who is Barbier’s wife. Burns held the title of Senior Vice President, Neuroscience, at Cassava Sciences prior to her departure, so she was officially an important scientific leader within the company. She was also implicated in the investigations on Wang as she was one of the co-authors of some of his research papers. The City University of New York, where Wang was primarily employed, published a report in 2023 on its investigation of Wang’s research. The university could not conclusively prove that Wang had falsified his studies but as part of the findings, Burns was found to bear responsibility for some of the possible faults in Wang’s work.
High risk… high reward
The controversies surrounding Cassava Sciences have had a massive impact on its stock price. Chart 1 below shows the company’s stock price from August 2021, the month in which the Citizen Petition was first raised, to 15 August 2024. In this period, Cassava Sciences’ stock price has fallen by around 70%.
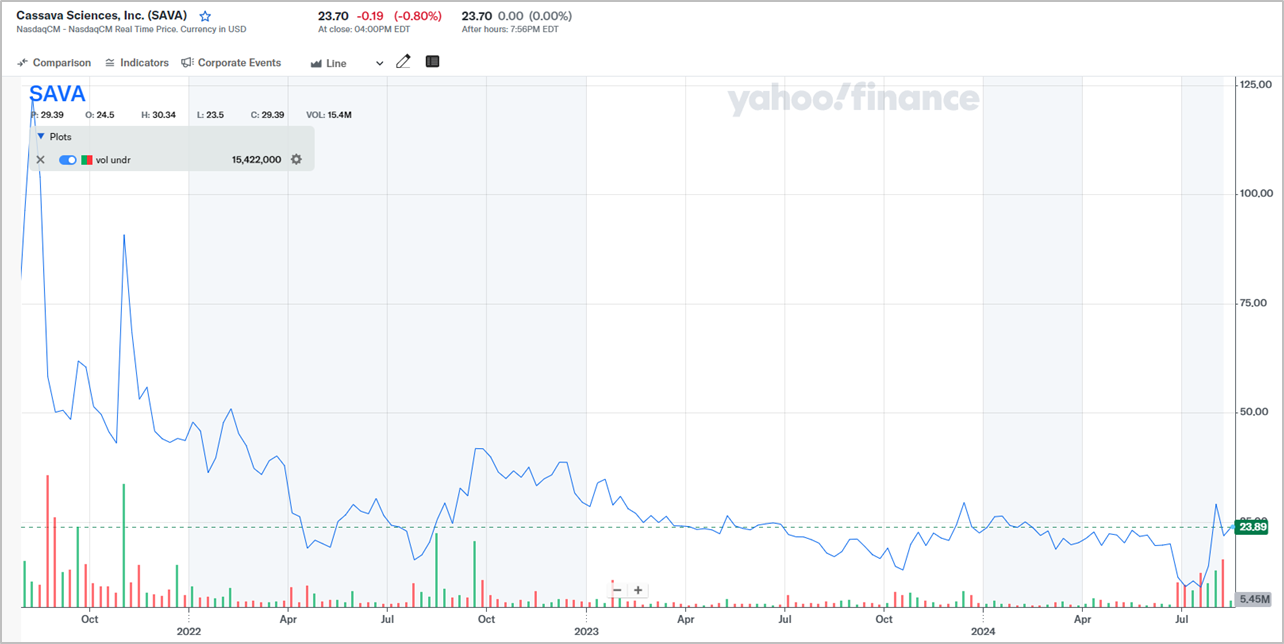
Chart 1; Source: Yahoo Finance
We invested in Cassava Sciences in early-August this year at an average price of US$32 per share. We believe the risk/reward profile is extremely favourable at this price. Here’s our simple valuation exercise:
- The latest data we had on Cassava Sciences’ share count at the time we invested was 47.98 million as of 8 May 2024 (the company’s 2024 second-quarter results filing, released a few days after our investment, show that its share count was also 47.98 million as of 5 August 2024). At a share price of US$32, Cassava Sciences thus had a market capitalisation of around US$1.5 billion.
- There are 6.5 million people in the US that suffer from AD, as mentioned earlier. If the price of simufilam is US$10,000 per year, the addressable market in the US alone is US$65 billion. For perspective, lecanemab and donanemab have annual prices of US$26,500 and US$32,000, respectively.
- If simufilam obtains FDA approval and captures just 20% of its addressable market in the US while Cassava Sciences earns a 20% net profit margin on the drug, the company would be looking at US$2.6 billion in annual profit. For a sanity check, the average net profit margins for Biogen (the biotech behind lecanemab) and Eli Lily (the biotech behind donanemab) for 2020-2023 are 21.4% and 20.6%, respectively. Throw in a 10-20 price-to-earnings ratio for Cassava Sciences, and the company could potentially be worth US$26 billion to US$52 billion, equivalent to 17-34 times the US$1.5 billion market capitalisation when we invested.
- We are not expecting Cassava Sciences’ stock price to immediately surge by 17-34 times if and when simufilam receives FDA approval. But if the drug is greenlighted, there is likely to still be a massive immediate jump in Cassava Sciences’ stock price, although the exact amount is anybody’s guess.
As highlighted previously in this thesis, there are definitely risks with Cassava Sciences. FDA approval for drugs undergoing Phase 3 trials typically happen with a low success rate of just 25%-30%. On the surface, allegations of fraud related to simufilam make the odds of FDA approval appear to be even lower. But there are a few things that are going in the favour of Cassava Sciences and simufilam, in our opinion.
The things in favour of simufilam
We acknowledge the risk that Wang could have tampered with data related to simufilam. But he had no input in collecting essential data points about the clinical symptoms of patients during simufilam’s clinical trials. The scope of his work only involved the collection and analysis of biomarkers. In addition, Wang’s input in the clinical trials was only in very early scientific research for simufilam and in the Phase 2B trial that was done before the extensive 24-month Phase 2 trial. The extent of Wang’s involvement is important because the 24-month Phase 2 trial is by far the most important clinical trial for simufilam to-date and provides more concrete data for the drug. As a reminder, the key findings of the 24-month Phase 2 trial are that the symptoms of AD patients improved or progressed slower, instead of changes in biomarkers. In our view, although biomarkers can be useful to indicate the severity of AD, the improvement and slowing of AD symptom-progression carries way more weight. An improvement in biomarkers without any corresponding improvement or slowdown of symptom progression is insufficient. We would prefer to see improvements in both biomarkers and AD symptoms, but the latter is likely the more crucial element for FDA approval, in our opinion. Our thoughts are backed by the fact that lecanemab, one of the human monoclonal antibody drugs for AD that received FDA approval recently, also had a Phase 3 clinical study whose primary endpoint was a slowdown in disease progression. Simufilam has so far shown to be effective in this regard in its Phase 2 clinical trials, and this is free from any allegations of manipulation.
There has also been progress with the SEC’s investigations on Cassava Sciences. If you recall, both the DoJ and the SEC were looking into the company. The DoJ’s probe has so far led to an indictment of Wang but the SEC has to-date not formally accused Cassava Sciences or Wang of any wrongdoing. Nonetheless, it seems like the SEC’s investigation will lead to fines levied on Cassava Sciences. On 8 August 2024, the company reported that it was in advanced discussions with the SEC to resolve the ongoing investigations and it had recorded a US$40 million loss contingency that is related to a potential settlement with the agency. This is a positive development for Cassava Sciences as it would allow management to put the SEC investigation behind and focus on simufilam’s Phase 3 trials and potential future commercialisation. US$40 million is a large sum of money for Cassava Sciences, but as we mentioned earlier, the company has enough financial resources to pay the settlement and fund the remainder of simufilam’s Phase 3 trials.
Then, there is the recent change of leadership at Cassava Sciences. Barbier may have been the founder of the company and its CEO for a long time, but in our view he has not been treating shareholders fairly. He had received multiple big paychecks despite the company failing to obtain FDA approval for its previous leading drug candidate, a painkiller known as Remoxy ER, before simufilam emerged. In 2018, Cassava Sciences, which was previously known as Pain Therapeutics, stopped development work on Remoxy ER; Barbier’s annual compensation from 2015 to 2017 ranged from US$1.9 million to US$2.4 million. Barbier also devised the aforementioned cash incentive plan that would see him (along with Wang and other personnel in the company) pocket large cash bonuses if the company’s market capitalisation crosses certain thresholds and stays there for at least 20 consecutive days. We think this form of compensation incentivises short-term thinking as there’s no consideration given to the company’s long-term business results. Rick Barry, who was appointed as Cassava Sciences’ new executive chairman and interim CEO just a month ago on 17 July 2024, has impressed us so far. His focus while running the company seems to be aligned with shareholders, which is getting FDA approval for simufilam. For example, since taking over the reins, he has dropped an ongoing defamation suit against a group of short sellers of Cassava Sciences’ shares. Cassava Sciences had also previously tried to sue two short sellers (who are not part of the aforementioned group) who submitted the Citizen Petition. We think dropping the lawsuit is a good move as it focuses the financial resources of the company and the energy of its people on what will actually matter to the company’s long-term health: The development of simufilam. As mentioned earlier, Cassava Sciences has also made progress in resolving the SEC’s investigation and this happened after Barry assumed leadership.
Since we’ve just been discussing Cassava Sciences’ management team, this is a good opportunity to bring up another important figure in the current set-up, namely, Chief Medical Officer, Dr James Kupiec. He joined the company in January 2021 and was appointed as Chief Medical Officer in December 2022. He previously worked for 17 years at pharmaceutical giant, Pfizer, and he led the design of simufilam’s Phase 3 trials. We have been impressed with him so far.
Finally, we think the aforementioned fact that 89% of eligible patients in simufilam’s Phase 3 trials have taken up the offer to continue on simufilam for the open-label extension trials is a positive indicator for the drug. Although the first top-line reading of data for the Phase 3 trials will only be shared sometime in December this year, the company’s willingness to extend the trial at this moment shows the company’s commitment to test simufilam extensively. Moreover, it’s also an indication that there is a demand for a new form of therapy for AD in the market.
Summary and allocation commentary
Cassava Sciences is not a typical investment in Compounder Fund’s portfolio to say the least. The company has no revenue, relies on shareholders’ funding for its research, is in the limelight for a host of wrong reasons, and only has one key drug candidate it is focusing on. If simufilam does not obtain FDA approval, Cassava Sciences would be worth effectively zero in our opinion.
But the upside potential is tremendous. Reasonable estimates of simufilam’s market penetration (should it be greenlighted by the FDA) and Cassava Sciences’ revenue and net profit lead to a value that is tens of times the size of the company’s market capitalisation at the time of our investment. Simufilam’s Phase 2 results were outstanding and Cassava Sciences has ample cash on its balance sheet (along with commitment from the new management team) to see through the drug’s Phase 3 clinical trials. Recent developments such as the announcement of the open-label extension trials also give us more confidence that a positive Phase 3 readout for simufilam is in sight. With a new leader at the helm, Cassava Sciences is also righting some of its past wrongs; the ongoing progress the company is having with the SEC’s investigation is a good example. At the price we invested in Cassava Sciences, we believe the potential returns could be pretty significant in just a few months if the drug’s RETHINK-ALZ Phase 3 trial’s top-line results, which are expected to be released by the end of this year, reads well. Moreover, the long-term gains could also be huge if simufilam is successfully commercialised and gains a commanding market share.
On the back of all this information, we decided to allocate around 0.9% of Compounder Fund’s portfolio into Cassava Sciences at our initial investment. We made Cassava Sciences a small position even though it has a highly asymmetric risk/reward profile, because there’s a material risk of the investment going to effectively zero very quickly if simufilam’s top-line results are poor or if the FDA eventually refuses to approve the drug.
And here’s an important disclaimer: None of the information or analysis presented is intended to form the basis for any offer or recommendation; they are merely our thoughts that we want to share. Of all other companies mentioned in this article, Compounder Fund only owns shares in Cassava Sciences. Holdings are subject to change at any time.

